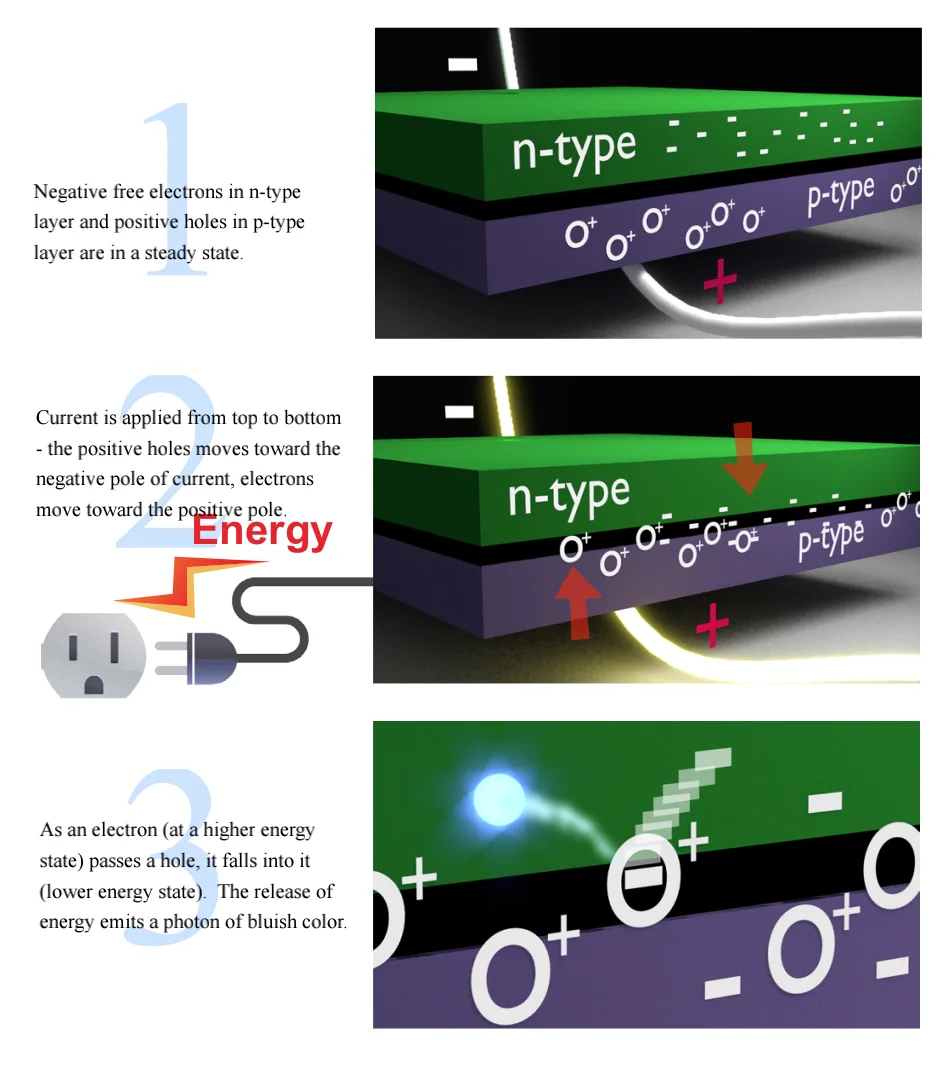
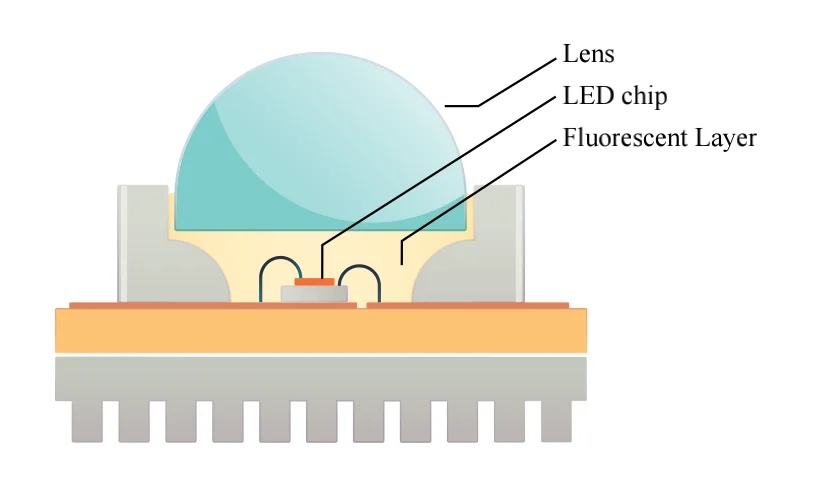
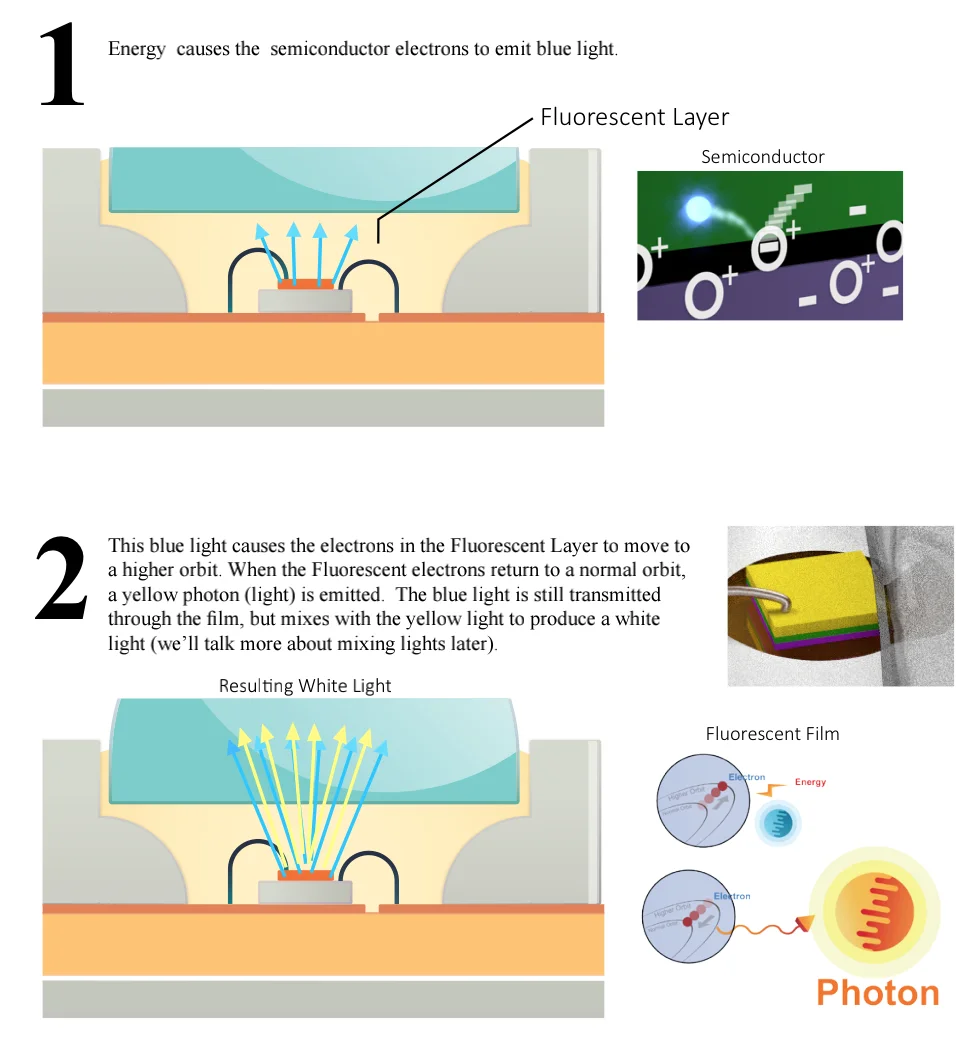
Electron movement in Semiconductors
As we saw on the previous page, electrons at higher orbits that drop back to lower orbits, release photons of light. In LED semiconductors, a similar strategy is at work.
Electrons jump from higher energy states to lower energy states.
LED semiconductors have two layers. The top semiconductor layer, called n-type, is modified to have extra free electrons. The bottom layer, called p-type, is also modified, but to have positively charged “electron holes” (but not really “holes”, more like pockets), which can attract free electrons.
When current is passed through these two layers, the electrons and holes move toward each other – when an electron is close enough to a hole, it falls into it, going from a high-energy state to a low state (hole) and as we already know, this releases a photon.